Innovative
and responsible Pharmaceuticals




16+
years of experiance in pharmacy
60+
export countries
10+
developed technologies
100+
workforce
1 million
annual sterile vials produced

Who are we

Vitālijs Skrīvelis
CEO and Member of the board
PharmIdea is a company certified in Good Manufacturing Practice by the European Union and internationally recognized for the production of sterile drugs, located in Latvia. We have more than 15 years of experience in the development of sterile drug technologies, preparation of documentation, and drug manufacturing. Our services include the development, production, registration of finished pharmaceuticals, and product sales in more than 60 international markets.
We specialize in the development and production of sterile drugs. The PharmIdea product range includes generic medications in therapeutic groups such as oncology, obstetrics, antifungal, cardiology, and anesthesia drugs. We pride ourselves on being able to find solutions for our clients’ individual needs by developing technology and preparing technical documentation in accordance with the requirements of authorities in many countries around the world, while maintaining the priority to ensure drug production according to European Union quality standards.
The company’s growth has been possible thanks to our team of more than 100 industry professionals. Together, we have created a friendly, open, and safe work environment that strives for improvement and excellence. PharmIdea constantly promotes individual growth and nurtures the talents of its employees to ensure the team comprises highly qualified, responsible, and in-demand professionals in the industry.
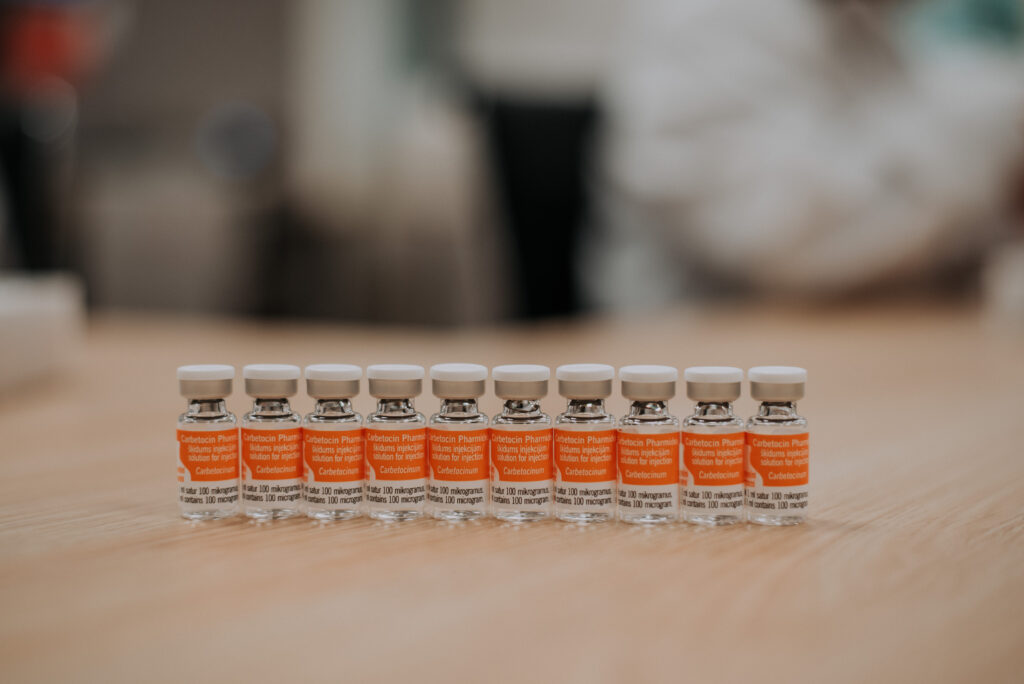



Sterile dosage forms
We develop and manufacture sterile drugs in vials, ensuring that our injectable products meet the highest quality standards across various therapeutic groups, including oncology, fungal infections, cardiology, anesthesia, and others.
Continuous development
We continuously monitor and adapt to the growing demands and current trends of the industry by improving technological processes, updating documentation, training staff, enhancing team competencies, and expanding our scope of operations.
From idea to sale
We provide a comprehensive full-service cycle for the development, production, preparation of registration documentation, and support during the process for sterile drug forms. We manufacture batches according to customer orders, performing complete analytical testing, quality control, and issuing certificates of compliance. We package, serialize, and deliver the finished pharmaceutical product to the customer in accordance with the registered documentation.




Development
We offer our clients the opportunity to complete the full product cycle, starting with the qualification of the active substance, development of technology, production of validation batches, stability studies, and preparation of documentation for drug registration in European and other markets.

MANUFACTURING
Pharmidea manufactures generic medications in sterile vials, utilizing various technological methods – lyophilization, aseptic filling, and autoclaving.

Licensing
We offer developed technical documentation for the registration of generic products that complies with EMA and ICH guidelines. During the drug registration process, we provide support and adjust the documentation in accordance with the requirements of the client’s country’s authorities.

INVESTIGATIONAL DRUG DEVELOPMENT AND MANUFACTURING
We have a license and experience in improving the formulation of investigational drugs, developing technology in the laboratory, and preparing documentation for Phase I-III clinical trials.
Take the next career step and join innovative pharmaceuticals
Join our team! We offer employment in a dynamic environment with ample opportunities to develop skills and advance qualifications for both seasoned industry professionals and emerging talents.

News
euroPLX 86 Munich
CPHI! At the heart of PharmaCPHI! A global community advancig human health
Read more CPHI! At the heart of PharmaCPHI! A global community advancig human health
Pharmidea at ACHEMA conference 2024
Career Day 2024
Certificates
”Good Manufacturing Practice" (GMP) certificate
The globally recognized GMP certificate shows high safety and control standards in all steps of the production process.
We have repeatedly obtained GMP opinion from more than 14 countries, including South Korea, Canada, Russia, United Arab Emirates.
partners

Latvijas Organiskās sintēzes institūts

Selectchemie Ag

Logenex Pharm GmbH

Viet Phap

Austell laboratories ltd

Kuwait Saudi Pharmaceutical Industries Co.

AV Medical CZ s.r.o.

Polfa SA Tarchomin

LIDDS AB

Find out more about our products, cooperation or career opportunities





